Rogacheva, O. N.; Izmailov, S. A.; Slipchenko, L. V.; Skrynnikov, N. R. A New Structural Arrangement in Proteins Involving Lysine NH3 + Group and Carbonyl. Scientific Reports 2017, 7 (1).
DOI: 10.1038/s41598-017-16584-y.
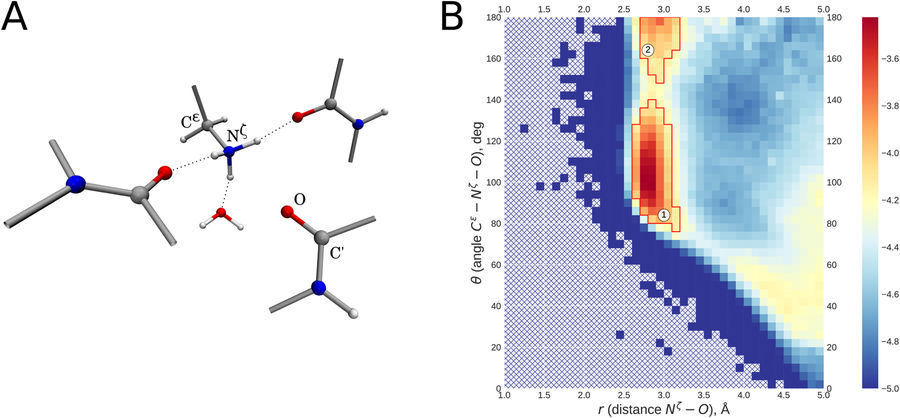
Screening of the Protein Data Bank led to identification of a recurring structural motif where lysine NH3+ group interacts with backbone carbonyl. This interaction is characterized by linear atom arrangement, with carbonyl O atom positioned on the three-fold symmetry axis of the NH3+ group (angle Cε-Nζ-O close to 180°, distance Nζ-O ca. 2.7-3.0 Å). Typically, this linear arrangement coexists with three regular hydrogen bonds formed by lysine NH3+ group (angle Cε-Nζ—acceptor atom close to 109°, distance Nζ—acceptor atom ca. 2.7-3.0 Å). Our DFT calculations using polarizable continuum environment suggest that this newly identified linear interaction makes an appreciable contribution to protein’s energy balance, up to 2 kcal/mol. In the context of protein structure, linear interactions play a role in capping the C-termini of α-helices and 310-helices. Of note, linear interaction involving conserved lysine is consistently found in the P-loop of numerous NTPase domains, where it stabilizes the substrate-binding conformation of the P-loop. Linear interaction NH3+ – carbonyl represents an interesting example of ion-dipole interactions that has so far received little attention compared to ion-ion interactions (salt bridges) and dipole-dipole interactions (hydrogen bonds), but nevertheless represents a distinctive element of protein architecture.
← PreviousТэги: Измайлов, Рогачева, Скрынников
